VIDEODROP

Phages
Analyze complex mixtures of phages for applications in ecology and phagotherapy. T4, P1 from 80 nm…
Demonstration
Measure in a single drop the concentration and the size of nanoparticles
in less than one minute ? Let’s go…
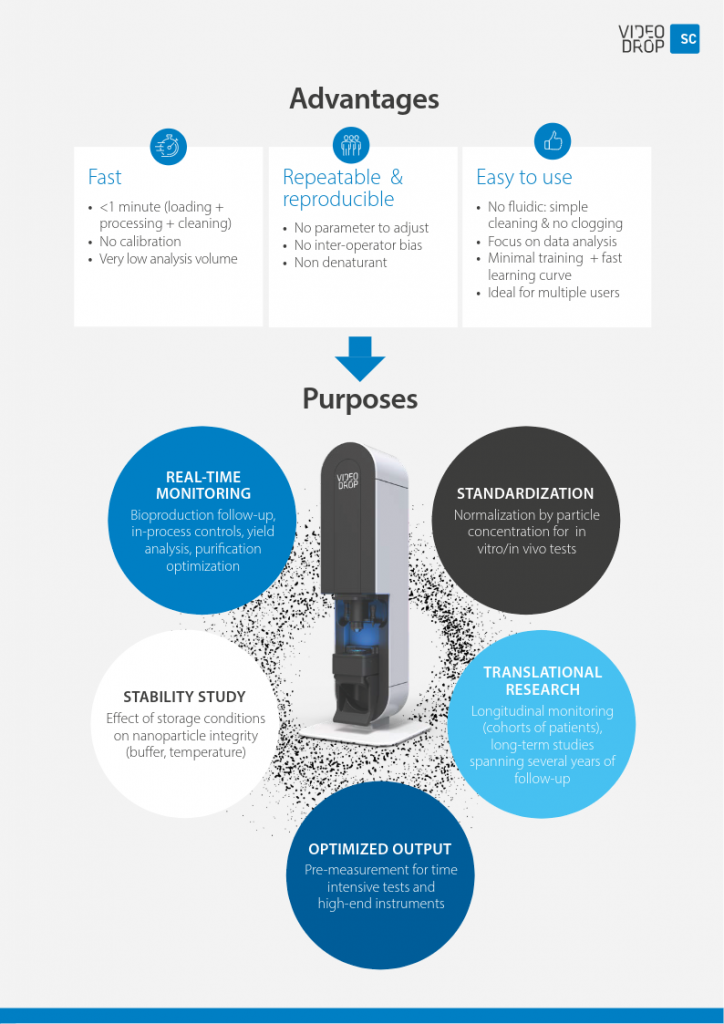
Fast, Reproducible, & Easy to use
Videodrop films in real time biological nanoparticles between 80 nm & 500 nm and provides a microscopic view up to 10µm in a drop. The compact device, assisted by a dedicated software, allows the manipulation to be very easy to perform. No labeling is necessary on the analyzed sample. It is possible to work on unpurified solutions and on little volumes (5µL) with a concentration range of 10^8 to 10^10 particles/ml.

Applications
A value proposition based on solid demonstrations
Videodrop, Performance qualification of a system measuring size & concentration of plastic nanoparticles
Technology
Based on the principles of interferometry, the Videodrop allows to « see » biological nanoparticles in the range of 80-500 nm.
The observation is done in 3 steps.
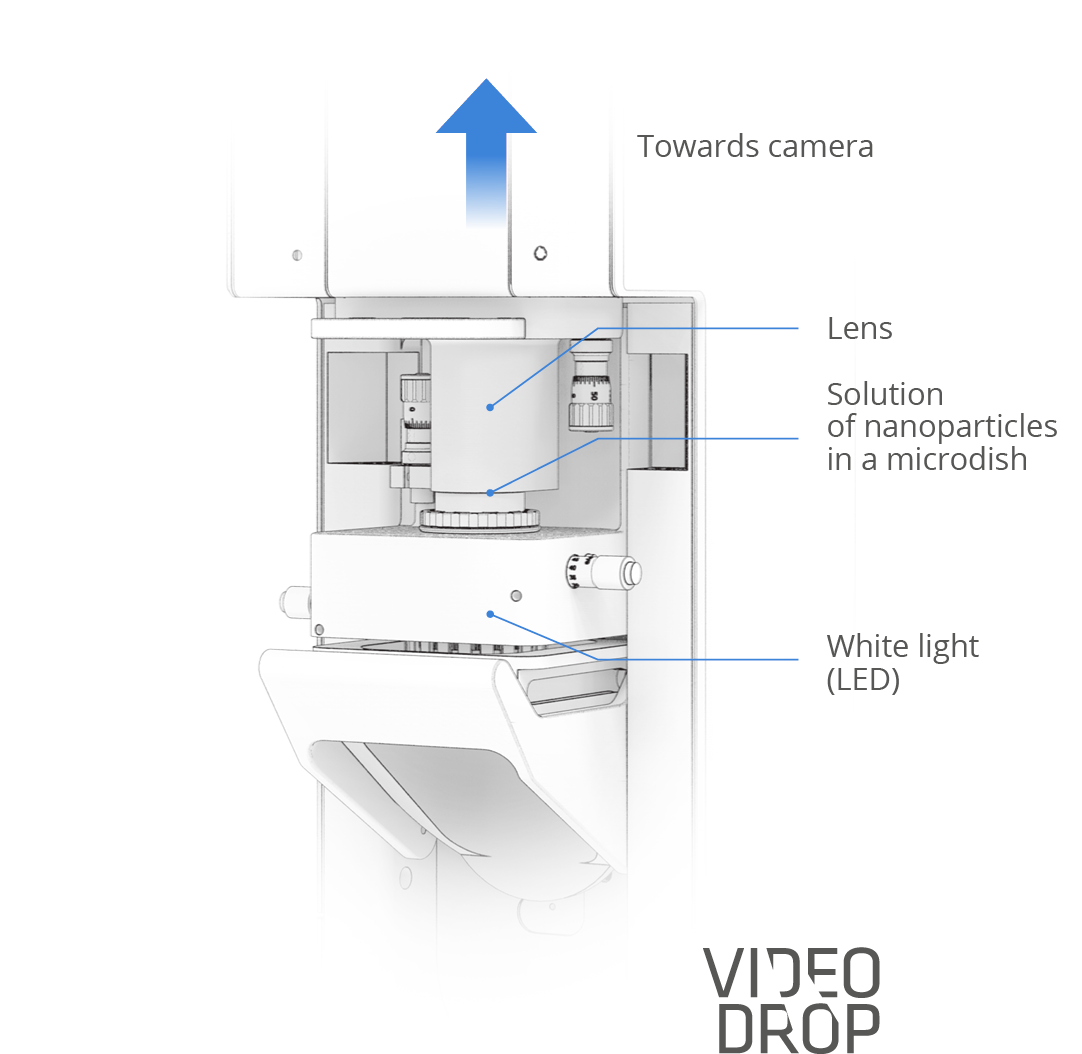
The device
A solution of 5 to 10µL containing nanoparticles is placed on a microwell lighted by a simple LED, while an optic system, coupled to a camera, films their movements.

The video
The resulting movie shows in real time the displacement of the nanoparticles.
A tracking algorithm allows to follow the trajectory of the observed objects.

Observation of a drop in the Videodrop:
Each black/white point is corresponding to the interferometric signal of a nanoparticle.

The Analysis
Thanks to its dedicated software, the calculation of speeds and distances of the trajectories enables to define the type, the size, and the number of observed elements.
The result is displayed on the user’s interface almost instantly.
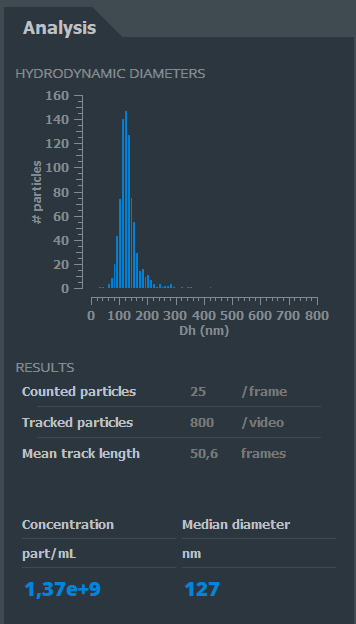
User’s interface of the Videodrop. The results of the analysis are displayed in real time.
ABOUT Myriade
Myriade is a French company created in 2017 that develops an innovative nanoscale imaging technology.

Myriade was born from the partnership between Quattrocento (Company builder, specialized in equipments for life sciences), Paris Sciences et Lettres, and the scientific team.
Based on the works of the Langevin Institute, a French academic laboratory specialized in optical and ultrasonic technologies for life sciences, the technology Videodrop relies on a single-arm interferometric technique.
It makes it possible to visualize without labeling living nanoparticles such as viruses, phages, or extracellular vesicles.
The process was developed by Professor Claude Boccara at the request of the Professor Martine Boccara, specialist in virology, who was looking for a quick method to list viruses present in seawater samples collected during the Tara Oceans expedition in 2013.
*The technology is protected by a family of patents.
TARA OCEANS
The aim of the Tara Oceans expedition was to take stock of marine plankton ecosystems (from viruses to fish larvae) in order to understand their functioning, but also to anticipate their relationship with climatic changes.
It sailed for 6 months and traveled 25 000 km to complete its mission. And on May 22, 2015, the prestigious journal Science was publishing the first five major scientific results of the expedition. Since then, hundreds of researchers from prestigious institutions analyze the collected samples and multiply the discoveries.

The boat Tara Oceans left Lorient on May 19, 2013, to come back on December 6 of the same year after having traveled 25 000 km.
THE OPERATIONAL TEAM

Philippe GARABEDIAN
Sales, Marketing & Operations

François MAZUEL
R&D, Production & Support

Lucas IONESCO
Business Developement

Donatien RAMIANDRISOA
R&D

Kevin AFFANNOUKOUE
R&D

Nicolas Rose
Applications & Support
THE SCIENTIFIC TEAM

Pr Martine Boccara
Virologist, Professor at the UPMC, former member of IBENS, researcher at the Muséum d’Histoire Naturelle.

Pr Claude Boccara
French physicist, specialized in optics. He is honorary scientific director of ESPCI and member of the scientific council of the Langevin Institute.
THE PUBLICATIONS
| Reference | Title | Institution | Type | Link |
|---|---|---|---|---|
| Boccara M, et al. | Full-field interferometry for counting and differentiating aquatic biotic nanoparticles: from laboratory to Tara Oceans. | Institut Langevin, ESPCI ParisTech, PSL Research University, Paris, France | Virus | Link |
| Turkki V, et al. | Experimental Evaluation of an Interferometric Light Microscopy Particle Counter for Titering and Characterization of Virus Preparations. | KCT, Kuopio Center for Cell and Gene Therapy, Finland | Lentivirus | Link |
| Reference | Title | Institution | Type | Link |
|---|---|---|---|---|
| Sabbagh Q, et al. | The von Willebrand factor stamps plasmatic extracellular vesicles from glioblastoma patients. | SOAP, CRCINA, Université de Nantes, France | EVs | Link |
| André-Grégoire,G., et al | Inhibition of the pseudokinase MLKL alters extracellular vesicle release and reduces tumor growth in glioblastoma. | SOAP, CRCINA, Université de Nantes, France | EVs | Link |
| de Poret, A., et al. | Extracellular vesicles containing the I-BAR protein IRSp53 are released from the cell plasma membrane in an Arp2/3 dependent manner. | CEMIPAI, CNRS Montpellier, France | EVs | Link |
| Sausset, R., et al. | Comparison of interferometric light microscopy with nanoparticle tracking analysis for the study of extracellular vesicles and bacteriophages. | INRAe, Jouy en Josas, France | EVs | Link |
| Troha, K., et al. | Autologous Platelet and Extracellular Vesicle-Rich Plasma as Therapeutic Fluid: A Review. | Laboratory of Clinical Biophysics, University of Ljubjanja, Slovenia | EVs | Link |
| Takada, A., et al. | Exploration of microRNA Biomarkers in Blood Small Extracellular Vesicles for Enzootic Bovine Leukosis. | Faculty of Applied Biological Sciences, Gifu University, Japan | EVs | Link |
| André-Grégoire,G., et al | Isolating plasma extracellular vesicles from mouse blood using size-exclusion chromatography, density gradient, and ultracentrifugation. | SOAP, CRCINA, Université de Nantes, France | EVs | Link |
| Reference | Title | Institution | Type | Link |
|---|---|---|---|---|
| Roose-Amsaleg C, et al. | Utilization of interferometric light microscopy for the rapid analysis of virus abundance in a river. | Institut de Biologie de l’Ecole Normale Supérieure (IBENS), PSL Research University, Paris, France | Phages | Link |
| Dugat-Bony E, et al. | Viral metagenomic analysis of the cheese surface: A comparative study of rapid procedures for extracting viral particles. | INRA, AgroParisTech, Université Paris-Saclay, Jouy-en-Josas, France | Phages | Link |
| Reference | Title | Institution | Type | Link |
|---|---|---|---|---|
| Ryo Kato, et al. | Highly Stable Polymer Coating on Silver Nanoparticles for Efficient Plasmonic Enhancement of Fluorescence. | Institute of Post-LED Photonics, Tokushima University | Silver nanoparticles | Link |
| Romolo, A., et al. | Assessment of Small Cellular Particles from Four Different Natural Sources and Liposomes by Interferometric Light Microscopy. | Laboratory of Clinical Biophysics, University of Ljubjanja, Slovenia | Evs, liposomes, spruce vesicles | Link |
| Jeran, M., et al. | Small Cellular Particles from European Spruce Needle Homogenate. | Laboratory of Clinical Biophysics, University of Ljubjanja, Slovenia | Spruce vesicles | Link |